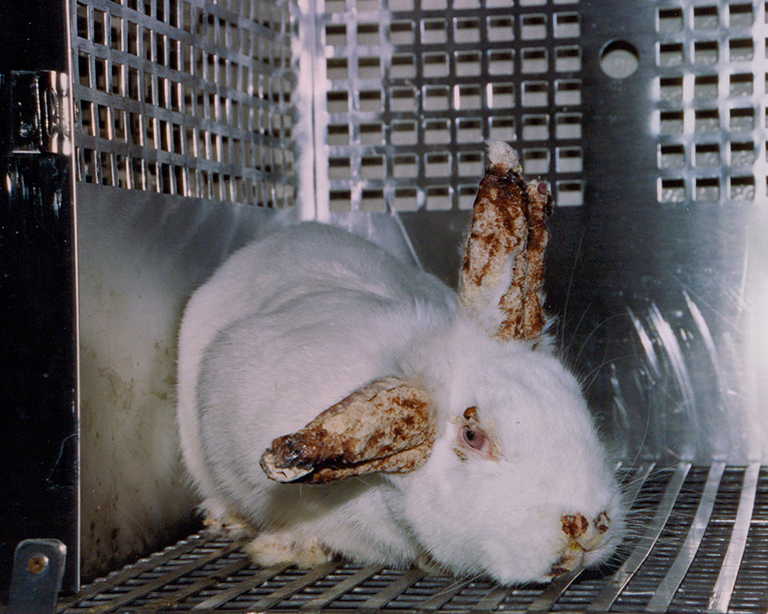
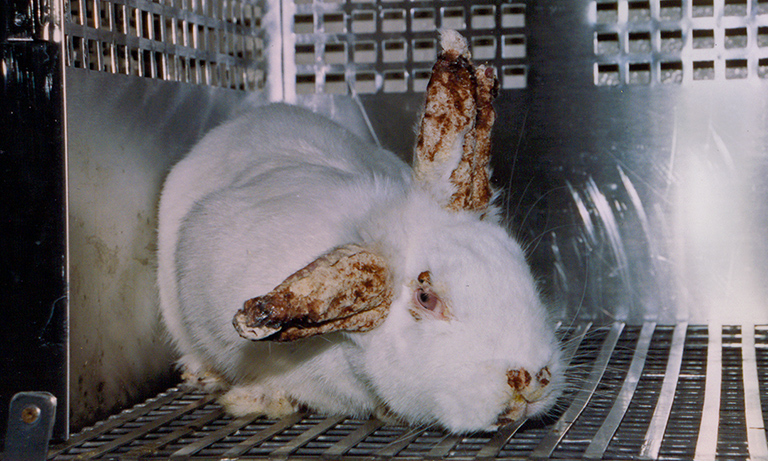
Multi-Billion $$ Lie
Data from animals artificially induced with human disease or injury is non-predictive. Researchers try to replicate findings in nonhuman subjects for decades — but often end up with info unrelated to human health or cures. Animals live in perpetual pain. Overdosed, burned, shocked, surgically mutilated, choked, beaten. Science can do better. Animal-free research tools are not an alternative. They are a better way, for animals and humans. If you care about taxpayer waste, human health, medical progress and animal cruelty… use your voice to end animal experimentation.
- Obstacles To Changing The Research Status Quo
- Medical Groupies: God-like reverence for doctors and all forms of medical research. White-coat heroes. Seen as gatekeepers to life-death.
- Media Taboo: Sacred-cow subject. Animal experiments exist beyond scrutiny in TV, film, news. I.E., YouTube bans Kinship Circle's video The Multi-Billion Dollar Lie in select countries and restricts viewing in the U.S. — simply for footage of animal experiments inside actual laboratories.
1 ECONOMIC WASTE: Government overspending, taxpayer waste, corruption. U.S. government annually spends an estimated $15 to $18 billion on animal research. Yet the animal model renders only 10% of new pharma products “safe” for market.1 Additionally, National Institutes Of Health (NIH) awards some $2.2 billion in contracts or grants to foreign entities for animal lab projects in 10 countries.2 Money is squandered on duplication of the same animal tests. “Right now, the private sector does half as much animal testing as government and taxpayer-funded university labs, but is responsible for 85% of FDA-approved medical innovations. This is just one example of inefficiency and waste in government-funded animal experiments.”3 By some estimates, U.S. agencies exhaust 50% of research funds on old-fashioned animal experiments — even though 89% of animal labs are not reproducible, a basic scientific tenet.4
- $3 Million: A decades-long Univ. of Wisconsin-Madison sound localization lab mangles cats to study how human brains process sound. Holes are drilled in a cat's brain to screw in a steel column. A second surgery opens a cat's head to destroy inner ears and lodge electrodes. The cat awakens deaf to undergo drills before killed to dissect her brain. “Funding violent experiments on cats means $3 million less is spent on research to actually improve human health. This can be conducted ethically with sophisticated brain imaging-recording techniques in humans.” Dr. Lawrence Hansen, neuroscience-pathology professor. Top 100 Brain Researchers, Journal of Alzheimer's Disease.5
- $5,075,798: Maternal deprivation tests in infant monkeys began (1960s) with Harry Harlow's Well Of Despair. Univ. of Wisconsin still isolates newborns with a live snake or wire “surrogate peer” to simulate adverse rearing conditions. They're further traumatized with human intruder skits, brain scans, spinal taps… that cause infant monkeys to self-mutilate before killed for brain dissection. The mom-baby bond is well-known. Yet studies have endured for a half century+.6
- Academic Research: A highly competitive grant culture creates pressure to churn out protocols, regardless of existing data or scientific validity. Schools get grants that help pay for overhead unrelated to labs. Quick money comes from animals, not clinical or cell-line studies. “NIH under-funds patient-oriented research,” with biggest cut for animal studies. Committee On Addressing Career Paths For Clinical Research.7
- Big Pharma & Academia, Million-Dollar Alliances: “Big Pharma and an academic institution agree jointly on which projects to fund, and in return for university financing, a company gets first option to commercialize results.”8
2 PROGRESS STALLED: Drug recalls, safety risks, no cures. The animal model presumes an effect in one species occurs in another. Yet science says genetic, metabolic, physiological and psychological traits vary significantly from species to species. A drug metabolized in a pig, dog or mouse doesn't look like the same drug in a person. Physiological pathways are unique in each species. In The Flaws and Human Harms of Animal Experimentation (NIH),9 author Aysha Akhtar footnotes myriad treatments that fail in humans due to “disparities between the animal experimental model and the human condition.”10
BASELINE FOR MEDICAL DEVELOPMENT?
Animal data creates false assumptions that speed new drugs from clinical trials to market, with unforeseen adverse drug reactions.
- Animal stroke experiments don't help humans. A rat, dog or primate can't replicate human physiology. “Accurately modeling disease in animals has proven to be an exercise in futility. Animals don't naturally develop atherosclerosis, a leading contributor to ischemic stroke. Researchers clamp their blood vessels or artificially insert blood clots. These interventions, however, do not replicate the elaborate pathology of atherosclerosis and its underlying causes. The inability to reproduce disease in animals so that it is congruent with human stroke has led to a high failure rate in drug development. Over 114 potential therapies initially tested in animals failed in human trials.”11
- Animal experiments misfire in drug design for cancer, amyotrophic lateral sclerosis (ALS), traumatic brain injury (TBI), Alzheimer's disease (AD), and inflammatory disorders. For cancer simulation in animals, tumors are unnaturally produced. This prototype can't “mirror the complex process of human carcinogenesis.” Useless or recalled cancer drugs rate among the highest of any disease category.12
- Over 20 ALS drugs, tested on animals, collapse in human trials. Riluzole, the sole FDA-approved drug for ALS, “shows notably marginal benefit on patient survival.”13 Animal prototypes for Traumatic Brain Injury likewise fail to recreate the intricacy of human TBI. Some 33 drugs appear to help animals artificially wounded in labs. All drugs fail in human trials.14
- Works in animal experiments: Approx 172 AD34 drugs and 150 inflammatory disease drugs. Fails in human usage: All 322 drugs that were forcibly dosed in animals. Mice, the go-to human disease model, supply 100% inaccurate data for sepsis, burns, trauma. Species discrepancy may render mice useless in cancer and heart disease tests as well. For sepsis (full-body inflammation from infection) investigators find that humans suppress a gene similar to a gene actively used in mice. When researchers disable this gene in lab mice, a test drug is successful. In humans, the same variable increases fatality. Study authors report “failure to block inflammatory responses in critically ill patients” across 150 clinical trials.15
- Today's drug fail rate hovers around 96%, up from an FDA calculated 92% in 2004. When drugs flop across the disease board, the problem begins with an “inability to adequately model human diseases in animals and the poor predictability of animal models… Lack of effectiveness and safety problems are not predicted by animal tests.”16, 17
- Animals exist in perpetual distress from handling, confinement, noise, isolation, pain, fear… Stress hormones influence animal data.18 Repeating the same experiments again and again, for decades, is a hard sell — given the lack of cures from animals induced with human disease/injury in settings humans never encounter.
- One study finds 47 of 53 landmark findings can't even be reproduced! “It was shocking. Pharmaceutical industry relies on these findings,” says Glenn Begley, former head of global cancer research at Amgen. Failure is blamed, in part, on animal models irrelevant to diseases, in an arena that fosters poor science, even fraud, as researchers fight for funds.19
3 HUMANS HARMED: Why do we justify animal cruelty for methods so flawed they even hurt people? The FDA cites Adverse Drug Reactions as the fourth leading cause of U.S. death. In hospitals, some 6.7% of patients experience ADRs, with 0.32% who die. This means over 2,216,000 people annually suffer ADR disability and 106,000 die. As the fourth top cause of death, ADRs surpass pulmonary disease, diabetes, AIDS, pneumonia, accidents, and automobile deaths. These stats don't account for ADRs in ambulatory backdrops or the estimated 350,000 ADRs in U.S. nursing homes each year. A precise ADR stat is unknown. All approved drugs undergo animal tests for “safety and efficacy.”20 Worldwide, ADRs are estimated at fourth to sixth place for cause of death. Animal-tested drugs now compete with heart disease, cancer and stroke — the very diseases they seek to cure — in “prevalent causes of mortality.”21
BIG BLUNDERS: WHEN DRUGS ENDANGER
When animal data misinforms, humans suffer. “Animal toxicity studies are poor predictors of toxic effects of drugs in humans.”22
- After decades and vast sums are spent on animal experimentation for HIV/AIDS vaccines, infamously on chimpanzees, all 90 animal-studied vaccines prove ineffectual in humans. Author Aysha Akhtar argues in her NIH article that “the implicit assumption that primate (and indeed any animal) data are reliable leads to significant and unjustifiable human suffering. Clinical trial volunteers for (HIV vaccine) gp120 were placed at unnecessary risk of harm.”23, 9
- When menopausal women on hormone replacement therapy (HRT) join the largest randomized study to date (1998-2002 Women's Health Initiative WHI)24, participants see a spike in coronary heart disease and breast cancer. The data triggers anxiety in HRT users and new restrictive prescriptions. WHI, along with another HRT study, abruptly end due to elevated stroke and breast cancer risk, undetected in animal tests.25
- Elan Pharmaceuticals abruptly ends a Phase 2 clinical trial after an Alzheimer's Disease vaccine induces brain swelling in humans. During animal experiments on GM mice and primates, no harmful impacts are observed. 26
- Volunteers dosed with TGN 1412, a drug to dampen the immune system, suffer grave (organ failure, brain edema) and permanent effects in under two hours, some in minutes. No ill effects are seen when tested in mice, rabbits, rats, Cynomolgus and rhesus monkeys. Monkeys endure lethal-dose toxicity tests, given 500 times the human dose.27 None show the instant catastrophic reactions observed in humans who get minute doses. “If experimentation using chimps and other nonhuman primates, our closest genetic cousins, are unreliable, how can we expect other animals to be reliable? The bottom line is that animal experiments, no matter the species, are highly unreliable — and have too little predictive value to justify the resultant risks of harms for humans,” concludes author Aysha Akhtar in her NIH National Library Of Medicine report.9
Valuable therapeutics for humans are discarded if unsuccessful in animals. “Treatments that fail to work or show some adverse effect in animals may be abandoned in preclinical testing even if they may have proved effective and safe in humans if allowed to continue through the drug development pipeline.”9
- Among 5,000–10,000 possible therapies studied in animals, just 5 or so make it to Phase 1 clinical trials. Pharma data is non-public, so the breadth of lost chances is unknown.28
- Tamoxifen, a crucial breast cancer drug, “would most certainly have been withdrawn from the pipeline if its propensity to cause liver tumor in rats had been discovered in preclinical testing rather than after the drug had been on the market for years.”29
- A drug for chronic myelogenous leukemia (CML) displays grave repurcussions in 5 species, with dogs who suffer acute liver malfunction. Liver damage does not occur in human cell assays. Clinical trials affirm the drug for human use, despite toxicity in animals.16 While leukemia patients luck out with a drug greenlit via human-based tests, others aren't as lucky. Common drugs such as aspirin (embryo toxicity in rats, rhesus monkeys), Tylenol (poisonous in dogs, cats) and penicillin (deadly in guinea pigs) might not exist if discovered under modern animal-test rules.30, 47
- Cyclosporine to treat autoimmune disease and deter organ transplant rejection, plus more pivotal drugs such as aripiprazole (Abilify) and esomeprazole (Nexium), are delayed or forfeited when animal findings show low viability. A PharmaInformatic report asks: How many other blockbuster drugs would be on the market today, if preselect compounds and drug-candidates didn't rely on animal tests?31 “These near-missed opportunities and the overall 96% failure rate in clinical drug testing strongly suggest the unsoundness of animal testing and provide powerful evidence for the need for a new, human-based paradigm in medical research… Repeatedly, researchers are lured down the wrong line of investigation from animal experiments that later prove to be inaccurate, irrelevant, or discordant with human biology.”9
BROKEN SYSTEM
Experimenters claim to extract some valid knowledge from animals because they are live beings. Yet if the science is fundamentally unreliable, with potential to harm humans, why do we defend a system that is inherently cruel? It's futile to debate experimenters themselves. In the lab, animals are viewed as a means to an end. Experimenters see themselves as “heroes” with noble careers. I.E., one told me I am not qualified to recognize animal cruelty, and thus my evidence is invalid. They speak condescendingly and paint anyone against the use of animals in research as an extremist.
A Better Way
A Better Way
HUMAN-FOCUS RESEARCH
4 Biotechnology has evolved with cellular, genomic and computational tools. Human biology tools mean researchers don't need to extrapolate info from animals to people. Guesstimates give way to a more predictive science. But “financial investment in human-based tech generally falls far short of investment in animal experimentation.”32, 9 Funding is needed to engineer and validate animal-free systems, plus advance models that simulate entire-body function — not just lone cells or organs. Animal-free research is not “alternative,” a word that implies animal tests are the “gold standard,” while human-relevant tools are less sophisticated. In fact, the reverse is true.
Lab-Grown Human OrgansOrganoids, a 3D in vitro cellular complex, come from pluripotent stem cells and adult stem cells. Organoids mirror authentic organs in formation, cellular structure, organ-wide configuration and most significantly, physiological actions. Brain, intestine, lung, liver and many more organs can be grown inside labs to replace inaccurate animal experiments for basic research, drug testing… “The field of organoid research can be regarded as an offshoot of stem cell research. The advent of induced pluripotent stem cells and supportive tech advances such as clonal culture and CRISPR/Cas9-based genetic manipulation now herald a new way of undertaking biological research and applying the outcomes.”33
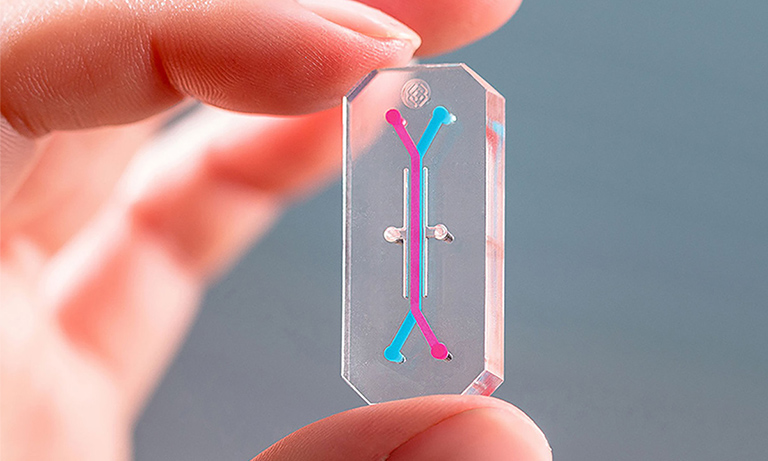
Organs-On-Chips“OoCs systems use engineered or natural miniature tissues grown inside microfluidic chips.” To emulate human physiology, chips regulate cell microenvironments and retain tissue-distinct roles.34 “The chip's goal is to lessen dependence on animal tests and decrease time and cost for developing drugs.” Instead of animal models, organ chips test chemicals and meds on human tissues, negating any errors.

3D-Printed OrgansSkin, bones, muscles, blood vessels, retinal tissue and some small-scale organs have already been 3D bioprinted. The technology harnesses a person's own cells to cultivate organs, and could save those who languish on organ donor lists. Right now, 3D bioprints can safety-test cardiac drugs in place of animal experiments. Another ALS-on-a-chip design explores remedies and causes of this little understood disease.35

Cognitive ComputingSystems that mimic human cognition employ Artificial Intelligence, data mining, machine learning and natural language processing — gleaned from real-time info. Vast folios of data are studied to broaden clinician insight for patient-specific treatments. Cognitive computing streamlines data for timely application. This speeds medical research, without time and money wasted on duplicative animal experiments.36
Human Toxome ProjectU.S. EPA tests chemicals for their capacity to act as endocrine disruptors. Some chemicals disturb hormone function, damaging animal growth and reproduction. Some 80,000 animals are killed in EPA toxicity screens, an “expensive, time-consuming and exorbitant use of animals” The Human Toxome Project, funded via NIH grant, advances animal-free modes to “deduce, validate and share molecular pathways of toxicity (PoT)”. A PoT public database could become the roadmap for toxicological research and regulatory testing.37

Genomics & BioinformaticsGenomics evaluates full genetic composition of an organism's genes (the genome) to learn the structure, function and evolution of genetic info. Bioinformatics, sophisticated computing and math, enables researchers to study huge quantities of DNA-sequence data to find variances that impact human health, disease or drug effects. Assimilation of informatics with genomic input can reveal data on entire populations and origins of disease. It can also better predict a treatment's efficacy or adversity, minus faulty animal experiments.38
DNA ChipsGenes or DNA fragments on a glass slide interact with a test drug to reveal which genes are activated or depressed. DNA chips facilitate an individualized medicine concept based on each person's different genetic blueprint. Scientists now do sweeping population studies I.E., to project frequency of breast cancer in individuals with a specific mutation or pinpoint gene sequence changes most ofen linked with certain diseases. “Microarrays can also be used to study the extent to which certain genes are turned on or off in cells and tissues.”39
Microfluidics ChipsMicroarray tech, a human-based standard lab tool, binds an array “to a solid surface. This chip is then bathed with DNA or RNA isolated from a study sample (such as cells or tissue). Microarray technology can be used for a variety of purposes in research and clinical studies, such as measuring gene expression and detecting specific DNA sequences.”40 For example, containers on a 2-cm wide chip each hold a tissue specimen. A test compound is added to a blood surrogate that circulates via mircrochannels for small-scale replication of the body's response. Chip sensors relay data for computer analysis. Hurel (Human RELevant) Corp. originally broke new ground in this method.
Human Tissue AnalysisAnimal response to a drug often fails to match that drug's interaction in people. Human tissue, sourced from surgical residual and biopsy materials or transplant tissue (via patient donors), facilitates human-based research on side effects prior to clinical trials.42 “In human tissue, we'll find answers to Alzheimer's, Parkinson's and other neurodegenerative diseases” (Dr. John Xuereb, Director, Cambridge Brain Bank and Wolfson Brain Imaging Centre). All viable data about HIV/AIDS, plus usable info on Alzheimer's and Parkinson's, comes from patient tissue analysis.
Computer ModelingA virtual human foretells drug metabolism and metabolite interaction for any organ. Computer modeling enables the molecular architecture of drugs to hone in on specific receptors. In mere minutes, scientists can reproduce experiments in silico to gain insight that takes years in a lab. During the COVID-19 pandemic a safe vaccine was needed quickly, casting light on the sluggish, inconclusive nature of animal tests. At the pandemic's apex, FDA analyzed computer simulations of acute respiratory distress syndrome (ARDS) from COVID-19 infection. “Based on our data, FDA approved an efficacy trial with a major pharma company without any animal models,” says David Harel, CytoReason CEO in Tel Aviv, Israel. Harel notes that computer models “can generate better predictions than animal models” and are “faster, cheaper, more accurate.”43
Advanced Magnetic Resonance ImagingMRI imaging tools — Functional Magnetic Resonance Imaging, Diffusion-Weighted Imaging MRI (DWI), Dynamic Contrast-Enhanced-MRI (DCE-MRI), BOLD-MRI, MR Spectroscopy (MRS), Radiomics — assess tissue pathologies “from degenerative and inflammatory diseases to cancer.” A pioneering field uses MRI to “exploit the diagnostic and therapeutic capabilities of nanocompounds.” Imaging tools retrieve info on nanconstructs and how they interact with diseases like cancer. “Nanoparticles allow the delivery of therapeutic agents to a target point… with antibody, peptide, or protein coatings that connect specifically to the surfaces of cancer cells.”44
More Human-Focused ToolsMany tools based in human biology have been around awhile, but are under-used. Functional In Vitro uses human-derived cells, cell lines, or cellular components to analyze cells and tissue. Microdosing assesses human metabolism data (metabolomics) for safe human trials earlier in product development. Autopsy/Biopsy analyzes full-body disease impacts post-mortem and amends common misdiagnoses. Epidemiology traces parallels within populations and has linked tobacco to cancer; cholesterol to heart disease; pregnancy folic acid deficit to spina bifida… Stem Cell Research, ethically sourced from donated adult and umbilical cord cells, has already remedied some cases of leukemia, heart attack recovery, Parkinson's. Post-Market Drug Surveillance regularly tracks new medical products to identify repercussions in a faster time frame. Wide-Scale Clinical Research is key to long-term efficacy of any drug or treatment.
Huge Win For AnimalsIn 2022, the FDA Modernization Act 2.0 is signed into law! Thanks to all who answered our call to action on this bill. Since its inception, Kinship Circle has fought to replace animal experiments with human-relevant research to speed medical progress. The new law ends a requirement to test all pharma goods on animals prior to human trials. Companies may now use human-focused tools instead of profoundly cruel and misleading animal labs. The Act also earmarks $5 million in funding for FDA's New Alternative Methods Program. Each time you act for animals — they are seen in a world that forgets them.
The FDA Modernization Act 2.0 ends a 1938 federal mandate that drugs must be tested on animals before they are used in human clinical trials. While the law doesn't ban animal testing, it allows drugmakers to use other methods, such as microfluidic chips and miniature tissue models… Advocates point to studies that have shown animal testing to be an unreliable predictor of toxicity in humans. And plenty of drugs work in mice but aren't effective in people. An estimated 90% of drug candidates in clinical trials never reach the market, and drugs that target the brain have an even higher failure rate. These inconsistencies, combined with the time, expense, and ethical issues associated with animal use, have led scientists to develop testing methods that aim to better recapitulate human physiology… The US Just Greenlit High-Tech Alternatives to Animal Testing.41
What Is ICCVAM? The Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) exists as a perpetual U.S. committee of National Institute of Environmental Health Sciences (NIEHS) to, in part, “reduce, refine, or replace the use of animals in testing where feasible.” Under the ICCVAM Authorization Act of 2000, 17 federal agencies “require, use, generate, or disseminate toxicological and safety testing information.” ICCVAM must validate human-focused (animal-free) research methods before they qualify as a step toward FDA drug/treatment approval.45 From 2000-2009 ICCVAM approved just 4 animal-free tests (out of 185 reviews): acute oral toxicity, dermal and ocular irritation/corrosion tests, and allergic contact dermatitis. By 2010, ICCVAM had gained U.S. and/or global regulatory acceptance for 17 animal-free tests. Comparatively, European Center for Evaluation of Alternative Methods (ECVAM) okayed 34 non-animal tests in 2009, with 170 more in evaluation. The European Commission has also phased out cosmetic animal tests.
ICCVAM's review process has been listless or non-active when federal offices such as Environmental Protection Agency or Food And Drug Administration seek validation for human-focused research tools. But under a 2013 change in leadership, Dr. Warren Casey (NICEATM) has steered ICCVAM in a more promising direction. “In 2018, ICCVAM published a strategic plan to replace the use of animals in testing. PETA scientists attended meetings to help develop this plan and submitted extensive comments. Implementing this roadmap has the potential to prevent millions of animals from suffering and dying in toxicity tests… NTP Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM) focuses on development and evaluation of human-based options to animal testing and offers counsel to ICCVAM. ICCVAM's task is to promote non-animal test methods for government-required testing.”46
Urge ICCVAM to further promote human-focus, non-animal testing
NTP Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM)
P.O. Box 12233, MD K2-16
Research Triangle Park, NC 27709
NICEATM phone: 984-287-3150
Nicole Kleinstreuer, PhD, Director, NICEATM: nicole.kleinstreuer@nih.gov
email: niceatm@niehs.nih.gov, ICCVAMquestions@niehs.nih.gov, iccvam@niehs.nih.gov, helena.hogberg-durdock@nih.gov, kamel.mansouri@nih.gov, david.allen@nih.gov, shagun.krishna@nih.gov
About ICCVAM
About NICEATM
National Institute of Environmental Health Sciences (NIEHS)
P.O. Box 12233, Mail Drop B2-06
Durham, NC 27709
NIEHS phone: 919-541-3201
Rick Woychik PhD, Director, NIEHS & NTP: NIEHSDirector@nih.gov
About NIEHS
National Institutes of Health (NIH)
9000 Rockville Pike
Bethesda, Maryland 20892
NIH phone: 301-496-4000
Lawrence Allan Tabak, DDS, PhD, Office Of The Director: lawrence.tabak@nih.gov
Tara Ashley Schwetz, PhD, Acting Principal Deputy Director: tara.schwetz@nih.gov
Research grants/funding questions: grantsinfo@nih.gov
NIH Staff Directory
NIH Institute & Center Contact Info
Food and Drug Administration (FDA)
10903 New Hampshire Ave
Silver Spring, MD 20993-0002
FDA phone: 1-888-463-6332
Robert M. Califf MD, FDA Commissioner of Food & Drugs: commissioner@fda.hhs.gov
Janet Woodcock MD, FDA Principal Deputy Commissioner: janet.woodcock@fda.hhs.gov
Peter W. Marks, Director, FDA Center for Biologics Evaluation & Research: peter.marks@fda.hhs.gov
Submit Questions, Comments: druginfo@fda.hhs.gov, Industry.Cosmetics@fda.gov, Industry.Biologics@fda.gov, Industry.Foods@fda.gov, Industry.Devices@fda.gov
More FDA Offices
Contact FDA
Contact Center for Drug Evaluation & Research (CDER)
Comment on FDA Proposed Regulations & Submit Petitions
Footnotes
- 1. Faunalytics. 3/14/18.
- 2. U.S. Government Accountability Office. 3/30/23, Fiscal year 2011-2021.
- 3. Justin Goodman. White Coat Waste Project.
- 4. Emily R. Trunnell, InsideSources.com. Newsday. 9/20/18.
- 5. Dr. Lawrence Hansen, neuroscience-pathology professor, University Of California-San Diego. Top 100 Brain Researchers, Journal of Alzheimer's Disease.
- 6. Harry Harlow Pit Of Despair. Vertical Chamber apparatus used in widely condemned experiments on rhesus macaque monkeys. University Of Wisconsin-Madison.
- 7. Committee On Addressing Career Paths For Clinical Research. National Academy Press, Bethesda, MD.
- 8. Morrison & Foerster LLP. Big Pharma and academia cement big deals in the quest for bigger prizes. Sharing an Umbrella. JDSUPRA 3/26/13.
- 9. NIH National Library Of Medicine. Aysha Akhtar. The Flaws And Human Harms Of Animal Experimentation. Camb Q Healthc Ethics. 2015 Oct; 24(4): 407–419. doi: 10.1017/S0963180115000079. PMCID: PMC4594046. PMID: 26364776.
- 10. NIH National Library Of Medicine. van der Worp HB, Howells DW, Sena ES, Poritt MJ, Rewell S, O’Collins V, et al. Can animal models of disease reliably inform human studies? Published online 3/30/10. doi: 10.1371/journal.pmed.1000245
- 11. NIH National Library Of Medicine. O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006 Mar;59(3):467-77.
- 12. NIH National Library Of Medicine. Robert AJ Matthews. Medical progress depends on animal models — doesn't it? Journal of the Royal Society of Medicine. 2008 Feb; 101(2): 95–98. • Mak IWY, Evaniew N, Ghert M. Lost in translation: Animal models and clinical trials in cancer treatment. American Journal in Translational Research. Published online 1/15/.
- 13. Nature Biotechnology. O'Sinha G. Another blow for ALS. Nat Biotechnol 31, 185 (2013). https://doi.org/10.1038/nbt0313-185
- 14. NIH National Library Of Medicine. O'Maas AI, Roozenbeek B, Manley GT. Clinical trials in traumatic brain injury: Past experience and current developments. Neurotherapeutics. 2010 Jan; 7(1): 115–126. doi: 10.1016/j.nurt.2009.10.022
- 15. Proceedings of the National Academy of Sciences. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. 2/26/13; 110(9): 3507–3512. doi: 10.1073/pnas.1222878110
- 16. South Texas Law Review. Pippin J. Animal research in medical sciences: Seeking a convergence of science, medicine, and animal law. 54 S. Tex. L. Rev. 469 (2012-2013)
- 17. NIH National Library Of Medicine. Hartung T, Zurlo J. Food for thought… alternative approaches for medical countermeasures to biological and chemical terrorism and warfare. ALTEX. 2012;29(3):251-60. doi: 10.14573/altex.2012.3.251.
- 18. Laboratory Animal Science. 2004.
- 19. Journal: Nature. C. Glenn Begley, former head of global cancer research at Amgen. March 2012.
- 20. U.S. Food & Drug Administration. Preventable Adverse Drug Reactions: A Focus on Drug Interactions. 3/6/18.
- 21. NIH National Library Of Medicine. Le Louët H, Pitts PJ. Twenty-First Century Global ADR Management: A Need for Clarification, Redesign, and Coordinated Action. Ther Innov Regul Sci. 2023 Jan;57(1):100-103. doi: 10.1007/s43441-022-00443-8. Epub 8/11/22. PMID: 35951160; PMCID: PMC9368697.
- 22. Slate. Allen A. Of mice and men: The problems with animal testing. 2006 June 1; last accessed 12/10/14. • NIH National Library Of Medicine. Heywood R. Target organ toxicity. Toxicol Lett. 1981 Aug;8(6):349-58. doi: 10.1016/0378-4274(81)90125-9. • Fletcher AP. Drug safety tests and subsequent clinical experience. Journal of the Royal Society of Medicine 1978;71:693–6.
- 23. NIH National Library Of Medicine. Bailey J. An assessment of the role of chimpanzees in AIDS vaccine research. Alternatives to Laboratory Animals 2008;36:381–428.
- 24. NIH National Library Of Medicine. Angelo Cagnacci, Martina Venier. The Controversial History of Hormone Replacement Therapy. Published 9/18/19. doi: 10.3390/medicina55090602. Medicina (Kaunas). 2019 Sep; 55(9): 602.
- 25. NIH National Library. Rossouw JE, Andersen GL, Prentice RL, LaCroix AZ, Kooperberf C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy menopausal women: Principle results from the Women's Health Initiative randomized controlled trial. JAMA. 7/17/02;288(3):321-33. doi: 10.1001/jama.288.3.321. • Andersen GL, Limacher A, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women's Health Initiative randomized controlled trial.* JAMA. 4/14/04;291(14):1701-12. doi: 10.1001/jama.291.14.1701.
*Kinship Circle note: Estrogen derived from pregnant mares (Premarin, Prempro) is sourced in cruelty. Mares are warehoused on pee farms, their male foals discarded.
- 26. NIH National Library Of Medicine. Lemere CA. Developing novel immunogens for a safe and effective Alzheimer's disease vaccine. Progress in Brain Research 2009;175:83. doi: 10.1016/S0079-6123(09)17506-4
- 27. NIH National Library Of Medicine. Attarwala H. TGN1412: From discovery to disaster. Journal of Young Pharmacists 2010 Jul;2(3):332–6. doi: 10.4103/0975-1483.66810.
- 28. International Rett Syndrome Foundation. Drug discovery pipeline. (accessed 9/24/14).
- 29. Follow the yellow brick road. Nature Reviews Drug Discovery 2003;2:167, at 167.
- 30. NIH National Library Of Medicine. For data on aspirin, see Hartung T. Per aspirin as astra…. Alternatives to Laboratory Animals 2009;37(Suppl 2):45–7 • See also note 16, Pippin 2013. • For data on penicillin, see Koppanyi T, Avery MA. Species differences and the clinical trial of new drugs: A review. Clinical Pharmacology and Therapeutics 1966;7:250–70 • See also Schneierson SS, Perlman E. Toxicity of penicillin for the Syrian hamster. Proceedings of the Society for Experimental Biology and Medicine 1956;91:229–30.
- 31. PharmaInformatic. Oral bioavailability of blockbuster drugs in humans and animals. (last accessed 9/19/14).
- 32. There is no direct analysis of the amount of money spent on animal testing versus alternatives across all categories; however, in 2008 Chronicle of Higher Education reported that funding of research involving animals (under basic research) of the National Institute of Health (NIH) remained steady at about 42% since 1990. See Monastersky R. Protesters fail to slow animal research. Chronicle of Higher Education 2008:54. In 2012, NIH director Francis Collins noted that support for basic research has held steady at 54% of NIH's budget for decades. The rest of NIH's budget is heavily funded toward clinical research, suggesting that preclinical human-based testing methods are much less funded. See also Wadman M. NIH director grilled over translational research centre. Nature News Blog 2012 Mar 20 (last accessed 3/5/15). No data suggests that NIH's funding of animal experimentation has decreased. A 2010 analysis estimates that at least 50% of NIH's extramural funding is directed into animal research; see Greek R, Greek J. Is the use of sentient animals in basic research justifiable? Philosophy, Ethics, and Humanities in Medicine 2010;5:14.
- 33. NIH National Library Of Medicine. Jaesang Kim. Lo and Behold, the Lab-Grown Organs Have Arrived! Int J Stem Cells. 2022; 15(1): 1–2. Published online 2/28/22. doi: 10.15283/ijsc22026
- 34. Leung, C.M., de Haan, P., Ronaldson-Bouchard, K. et al. A guide to the organ-on-a-chip. Nature Reviews Methods Primers 2, 33 (5/12/22). https://doi.org/10.1038/s43586-022-00118-6
- 35. Carolyn Barber. 3D-printed organs may soon be a reality. Looking ahead, we'll not need donor hearts. Fortune Well. 2/15/2023
- 36. Julie Clements. How Cognitive Computing in the Healthcare Industry Can Help Improve Patient Care. 28-May-2018. Last modified on 1/23/23. Medical Transcription Company. • Srivani M, Abirami Murugappan, Mala T. Cognitive computing technological trends and future research directions in healthcare. Review Artif Intell Med. 2023 Apr;138:102513. doi: 10.1016/j.artmed.2023.102513. Epub 2/23/23.
- 37. Bouhifd M, Andersen ME, Baghdikian C, Boekelheide K, Crofton KM, Fornace AJ Jr, Kleensang A, Li H, Livi C, Maertens A, McMullen PD, Rosenberg M, Thomas R, Vantangoli M, Yager JD, Zhao L, Hartung T. The human toxome project. ALTEX. 2015;32(2):112-24. doi: 10.14573/altex.1502091. Epub 2015 Mar 4. • (Hartung and Rovida 2009; Hartung 2011).
- 38. National Human Genome Research Institute. David Adams, M.D., Ph.D. Office of the Clinical Director, NHGRI. Bioinformatics, as related to genetics and genomics. updated: 7/1/2023. • The Jackson Laboratory 2023 Genetics vs. Genomics.
- 39. DNA Microarray Technology Fact Sheet. NIH National Human Genome Research Institute. 8/15/20
- 40. Microarray Technology. NIH National Human Genome Research Institute. 7/1/23
- 41. Emily Mullin. The US Just Greenlit High-Tech Alternatives to Animal Testing. Wired Science. 1/11/23
- 42. Roland Linder. What is Human Tissue Testing? REPROCELL. 3/14/22
- 43. Brian Buntz. When can computer models replace animal trials? Drug Discovery & Development 4/8/21
- 44. Bruno F, Granata V, Cobianchi Bellisari F, Sgalambro F, Tommasino E, Palumbo P, Arrigoni F, Cozzi D, Grassi F, Brunese MC, Pradella S, di S Stefano MLM, Cutolo C, Di Cesare E, Splendiani A, Giovagnoni A, Miele V, Grassi R, Masciocchi C, Barile A. Advanced Magnetic Resonance Imaging (MRI) Techniques: Technical Principles and Applications in Nanomedicine. NIH National Center For Biotechnology Information. Cancers (Basel). 3/23/22;14(7):1626. doi: 10.3390/cancers14071626.
- 45. NIEHS. About ICCVAM. 5/18/23
- 46. PETA. Progress! Feds Have a New Roadmap to Reducing Experiments on Animals. 9/25/19. • National Toxicology Program.
- 47. NIH National Library. Gail A. Van Norman, MD. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach?. JACC Basic Transl Sci. 11/25/2019;4(7):845-854. doi: 10.1016/j.jacbts.2019.10.008.
Disaster aid for animals + action for all hurt by greed, cruelty, hate.
KINSHIP CIRCLE2000
info@kinshipcircle.org314-795-2646
7380 KINGSBURY BLVD
ST. LOUIS MO 63130
KinshipCircle.org
PRIVACY POLICY
SITE DESIGN: BRENDA SHOSS